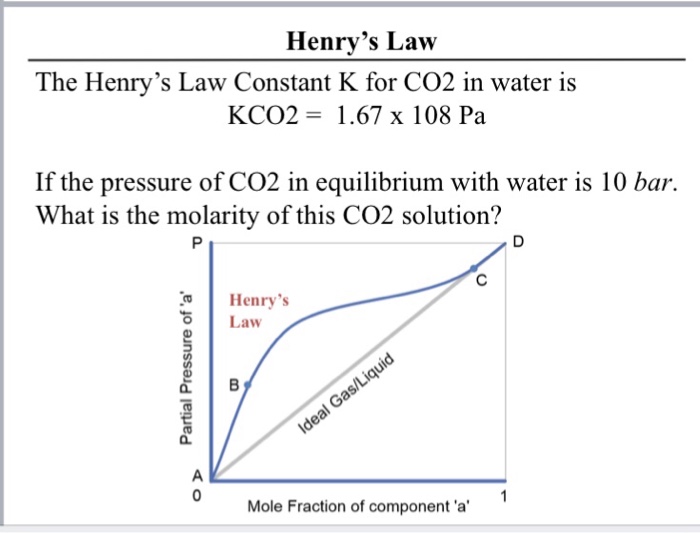
Henry's law constant for CO2 in water is 1.67 x 10^8 Pa at 298 K. - Sarthaks eConnect | Largest Online Education Community

Natural Logarithm of Henry's Law Constant for the Adsorption of CH 4 in... | Download Scientific Diagram

Henry's law constant for CO2 in water is 1.67 × 10^8 Pa at 298K. Calculate the quantity of CO2 in 500 ml of soda water when packed under 2.5 atm CO2 pressure at 298 K.
Henry's law constant of CO2 in water at 298 K is 5/3 K bar. Determine its concentration (mole fraction) in rain if CO2 is 1

Henry\'s law constant for `CO_(2)` in water is `1.67xx10^(8) Pa` at `298 K`. Calculate the quant... - YouTube

Henry's Law constant for CO2 in water is 1.67 × 10^8 Pa at 298 K. Calculate the quantity in 1 L of soda water when packed under 2.5 atm pressure at 298 K.
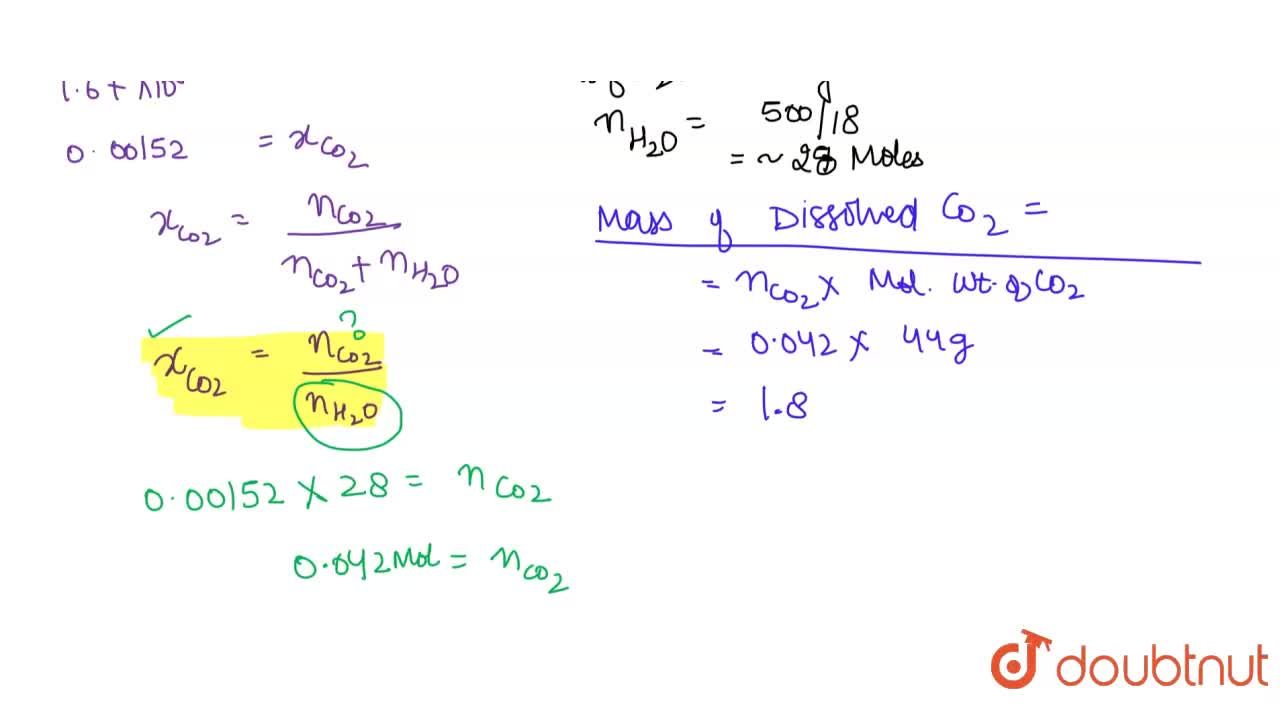
Henry's law constant for CO(2) in water is 1.67xx10^(8) Pa at 298 K. Calculate the quantity of CO(2) in 500mL of soda water when packed under 2.5atm CO(2) pressure at 298 K.
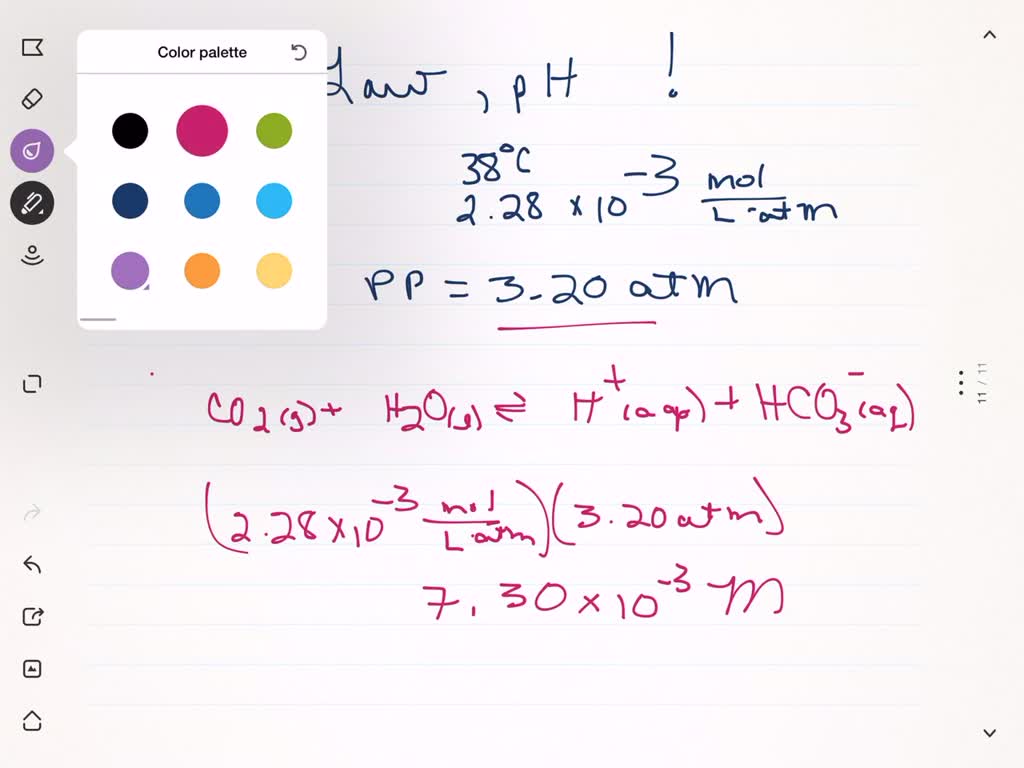
SOLVED:Henry's law constant for CO2 at 38^∘ C is 2.28 ×10^-3 mol / L ·atm. Calculate the pH of a solution of CO2 at 38^∘ C in equilibrium with the gas at
![Henry's law constant for CO(2) in water is 2.5xx10^(8)Pa at 298K. Calculate mmole of CO(2) dissolved in 14g water at 2.5atm pressure at 298K. [Take 1atm=10^(5)N//m^(2) or Pa] Henry's law constant for CO(2) in water is 2.5xx10^(8)Pa at 298K. Calculate mmole of CO(2) dissolved in 14g water at 2.5atm pressure at 298K. [Take 1atm=10^(5)N//m^(2) or Pa]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/16113056_web.png)
Henry's law constant for CO(2) in water is 2.5xx10^(8)Pa at 298K. Calculate mmole of CO(2) dissolved in 14g water at 2.5atm pressure at 298K. [Take 1atm=10^(5)N//m^(2) or Pa]
Henry's law constant for CO2 in water is 1.67 × 108 Pa at 298 K. Calculate the quantity - Sarthaks eConnect | Largest Online Education Community

Henry's law constants and diffusivities for gases in water at 37 °C a . | Download Scientific Diagram

Henry's Law constant for CO2 in water is 1.67 × 10^8 Pa at 298 K. Calculate the quantity in 1 L of soda water when packed under 2.5 atm pressure at 298 K.

Henry's law constant for CO2 in water is 1.678 * 10^8 pa at 298K the quantity of CO2 in 500ml of soda water when packed under 2.5 atm pressure is :