acid, hydrochloric acid, and citric acid. Dilution factor is calculated... | Download Scientific Diagram

Calculating molarity units molar concentration of solutions practice questions on molarity how to make up a standard solution how to determine solubility gcse chemistry igcse KS4 science A level GCE AS A2

Calculate the pH of the following solutions : 1 mL of 13.6 M HCl is diluted with water to give 1 litre of solutions.






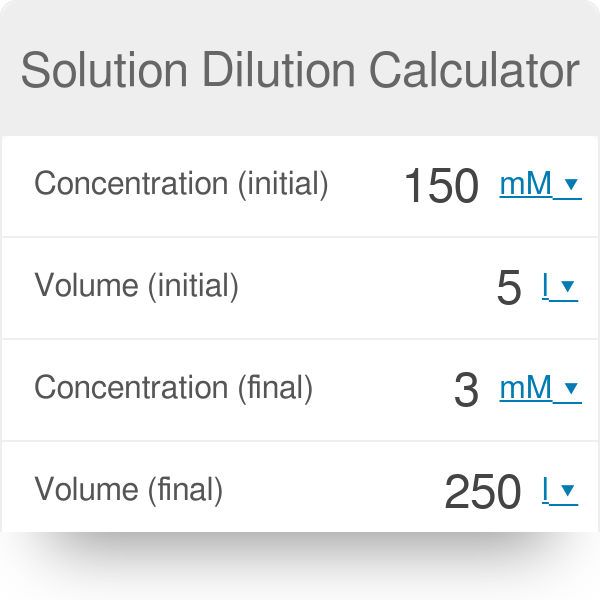
![Solution Dilution Calculator - [100% Free] - Calculators.io Solution Dilution Calculator - [100% Free] - Calculators.io](https://calculators.io/wp-content/uploads/2018/05/Solution-Dilution-Calculator.png)

:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)